
U.S. Influenza Diagnostics Market Size and Forecast, By Test (RIDT, RT-PCR, Cell Culture), By End-use (Hospitals, Laboratories, Point-of-Care Testing), And Trend Analysis, 2013 - 2024
- Published: September, 2018
- Format: Electronic (PDF)
- Number of pages: 66
- Industry: Healthcare
Industry Insights
The U.S. influenza diagnostics market size was valued at USD 546.2 million in 2016. Growing prevalence of influenza and demand for faster disease diagnosis are expected to foster market growth over the forecast period. In addition, new product developments and technological advancements in healthcare sector are expected to fuel the growth over the projected period.
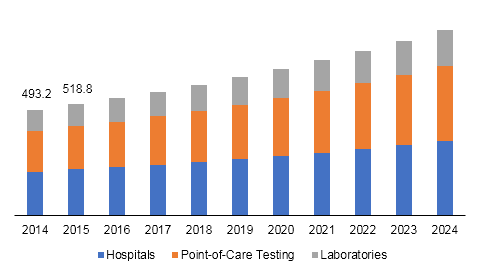
U.S. influenza diagnostics market size, by end-use, 2014 - 2024 (USD Million)
For instance, in 2014, Lab-in-a-Tube influenza A/B assay was developed by Massachusetts-based IQuum. In addition, BioFire Diagnostics, LLC, developed FilmArray diagnostic system to detect bacteria, viruses, parasites, and yeast. Rising prevalence of chronic health conditions, such as respiratory diseases, heart and blood disorders, along with government initiatives to control the disease prevalence are expected to drive the market in near future.
Heart diseases, respiratory disorders, asthma, blood disorders, and diabetes are some of the lifelong health conditions. Adults in the age group 65 years and above are at high risk of developing flu. As per statistics, more than 40% of the U.S. population was suffering from at least one chronic disease in 2016. People suffering from chronic diseases are at a higher risk of contracting the flu. Thus, growing cases of the disease are anticipated to boost the regional market over the forecast period.
The World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) actively record data related to influenza virus to counter the disease in a systematic manner. Agencies are working towards achieving goals set by the government to achieve better health for all people. For example, Healthy People 2020, an initiative launched by the U.S. Department of Health and Human Services (HHS) is focused on promoting national health and preventing chronic diseases in the country.
In 2013, CDC’s vaccination coverage was successful in achieving 90% of the target. These organizations are not only working in their respective nations but are also working in consensus with other national bodies to take effective measures for disease prevention. Growing health awareness among people regarding early diagnosis of flu related symptoms is expected to drive the U.S. influenza diagnostics market.
Segmentation by Test
• Rapid Influenza Diagnostic Test (RIDT)
• Reverse Transcription Polymerase Chain Reaction (RT-PCR)
• Cell Culture
• Others
Rapid influenza diagnostic tests segment led the market in 2016. Collection of respiratory tract specimens early in the illness with specificity and high sensitivity is expected to drive the market over the forecast period. These tests are immunoassays and are the most preferred diagnostic tests available for influenza. In addition, chances for negative results are very low in RIDT. All these factors are contributing to expansion of the segment.
RT-PCR tests are influential tests and can specifically determine the type A and B of the influenza virus. The cell culture segment is driven by its usage on testing prevalence rate of influenza. Cell culture are standard procedures for virus isolation owing to technological advancement. The ability to precisely identify the disease virus is expected to fuel the cell culture segment demand over the projected period.
Segmentation by End-use
• Hospitals
• Point-of-Care Testing
• Laboratories
The hospitals segment led U.S. influenza diagnostics market in 2016. Increased awareness regarding chronic diseases and easy availability of healthcare facilities are expected to drive the segment in near future. Point-of-care testing is expected to be the fastest-growing segment due to ease-of-use offered by these tests. These tests can be used by anyone, as they do not require much expertise, except basic operational knowledge.
Factors such as efficient work flow process, speed of diagnosis and treatment, expanded testing capabilities, ease of handling, and specimen stability are expected to drive the segment further. Importance of laboratory diagnosis of influenza has increased for containment, prevention, surveillance, and treatment of the associated illnesses. Emergence of Highly Pathogenic Avian Influenza (HPAI) viruses has prolonged the role of laboratory to include containment and isolation of the virus for disease observation and vaccine development.
Competitive Landscape
The companies operating in the market are focusing on the development of cost-effective and technologically advanced devices to initiate effective treatment. For instance, Alere Inc. received clearance from the U.S. Food and Drug Administration (FDA) for Alere i Influenza A & B test. This test has a faster diagnostic efficiency and the capability to identify influenza A and B viruses in a span of less than 15 minutes. Some of the key companies operating in the U.S. influenza diagnostics market include Alere, Inc.; Becton, Dickinson and Company (BD); Meridian Bioscience, Inc.; Quidel Corporation; Roche Diagnostics Corporation; SA Scientific; Sekisui Diagnostics; and Thermo Fisher Scientific, Inc.

Choose License Type
- World's largest premium report database
- Transparent pre & post sale customer engagement model
- Unparalleled flexibility in terms of rendering services
- Safe & secure web experience
- 24*5 Research support service
